
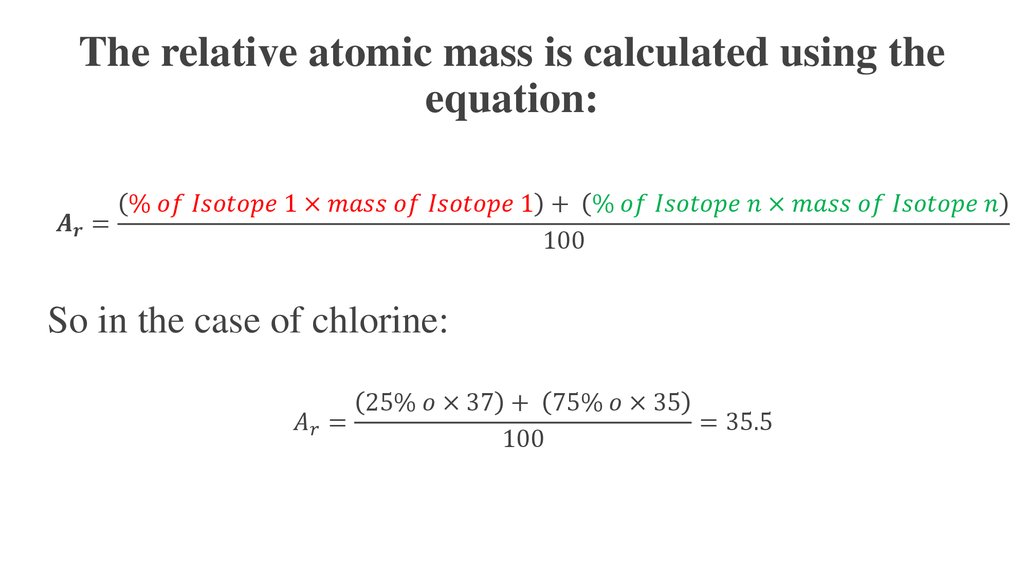
Therefore, the atomic mass of the element that has the atomic number 36 and neutrons 32 is 68. The mass of neutrons is 32 and the mass of protons is equal to the atomic number of a given element, so it will be 36.Ītomic mass of the element = 36 + 32 = 68 The atomic mass of an element that has the atomic number 36 and neutrons 32? The Einstein mass energy conversion equation m E/c2is the basis for converting mass (kg) to other measures of energy in consistent SI units. Therefore, the atomic mass of the element that has the atomic number 22 and neutrons 24 is 46.Ģ. Atomic mass, the mass of a given atom or molecule, can be expressed in standard SI mass units - grams, kilograms. The atomic mass of the element = Mass of protons + mass of neutronsĪtomic mass of the element = 24 + 22 = 46 1.Understand how atomic mass is represented. The mass of neutrons is 24 and the mass of protons is equal to the atomic number of a given element, so it will be 22. The atomic mass of an element that has the atomic number 22 and neutrons 24? Average atomic mass can also be calculated by the sum of the atomic mass of an isotope, multiplying each by the relative abundance of different isotopes of the element and dividing by the sum of the relative abundance of isotopes of the element.ġ.Another way of calculating atomic mass is by the sum of the atomic mass of protons and neutrons.First, with the help of the periodic table, we can find out the atomic mass of any element.The Formula for Computing Atomic MassĪtomic mass can be calculated in three ways. It is calculated by the average mass of the isotope of that element divided by the mass of the Carbon – 12 atom. Again, in this equation, the elements havent been conserved, by the mass number and proton number have 14 14 +0, and 6 7 1. We can determine the relative atomic mass of an atom also with the help of atomic mass. As you can see the elements havent been conserved, but the mass number and proton number have 6 + 2 2 4, and 3 + 1 2 2. Atomic mass can be calculated by the sum of the mass number of protons, electrons, and neutrons. One amu is defined as one-twelfth of the mass of the C 12 carbon atom. The standard unit of atomic mass is kilogram while the non-standard unit of atomic mass is amu denoting atomic mass units. What is Atomic Mass?Ītomic mass is defined as the mass of the atoms that are present in any molecule or compound. This article will enable the learners to compute the atomic mass of an atom of an element with conceptual clarity. However, the atomic and mass numbers stay the same.The Atomic mass formula is a measure to find out the mass of one atom of an element. When doing calculations, you need to bear in mind that the number of electrons does not equal the number of protons. Since 1961, it has been defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. An anion is a negative ion is when an atom gains electrons so it contains more electrons than protons.Ītomic Structure – Atomic and Mass Number.A cationa positive ion is formed when an atom has lost electrons, after which it contains less electrons than protons.Ions are formed from atoms when electrons are transferred between them: Number of electrons = number of protons (in an atom).Number of neutrons = mass number – atomic number.We can perform calculations to determine the number of subatomic particles. The mass of an atom can be calculated/ measured by adding both the number of protons and neutrons and then multiplying the result by 1 am.
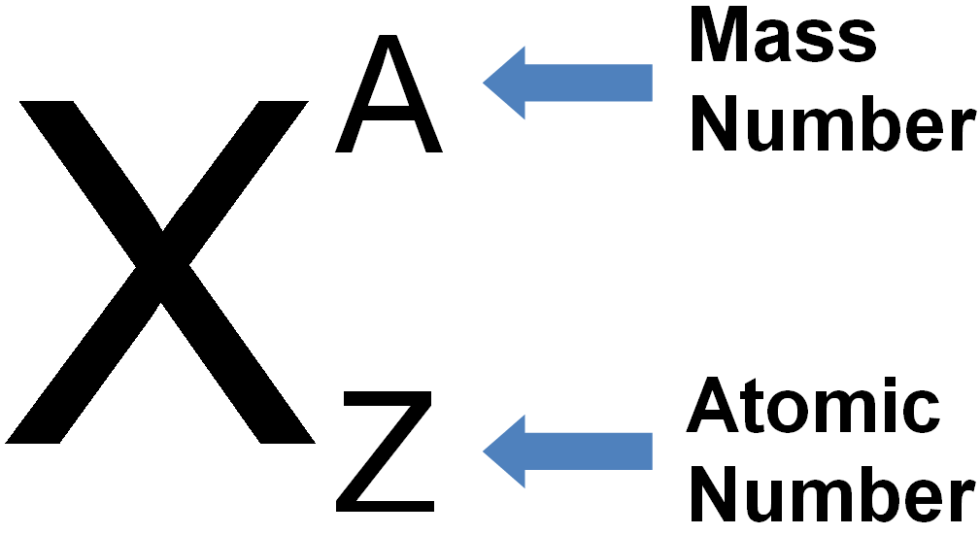
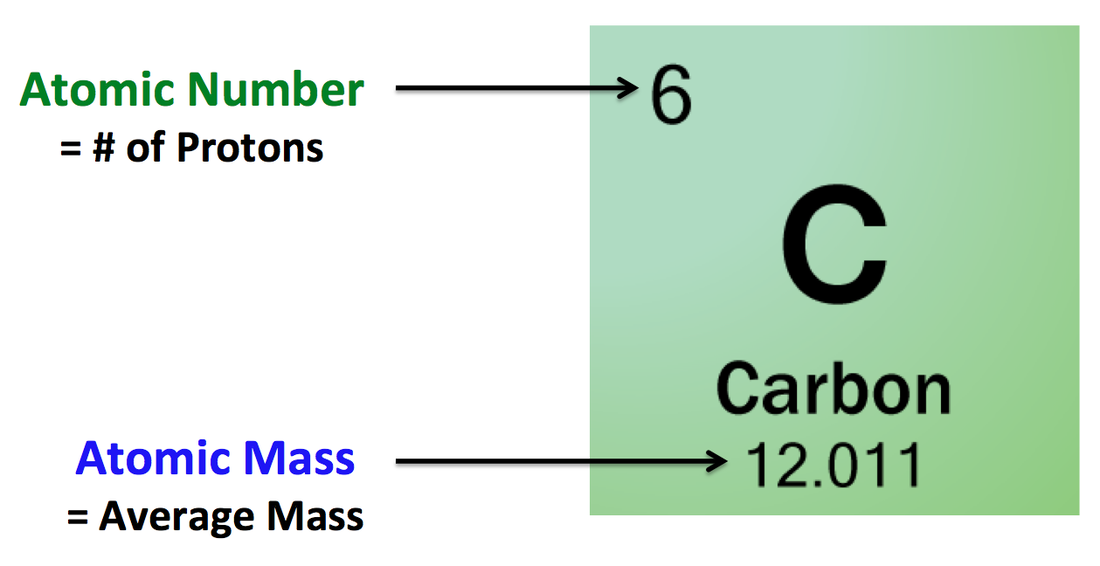
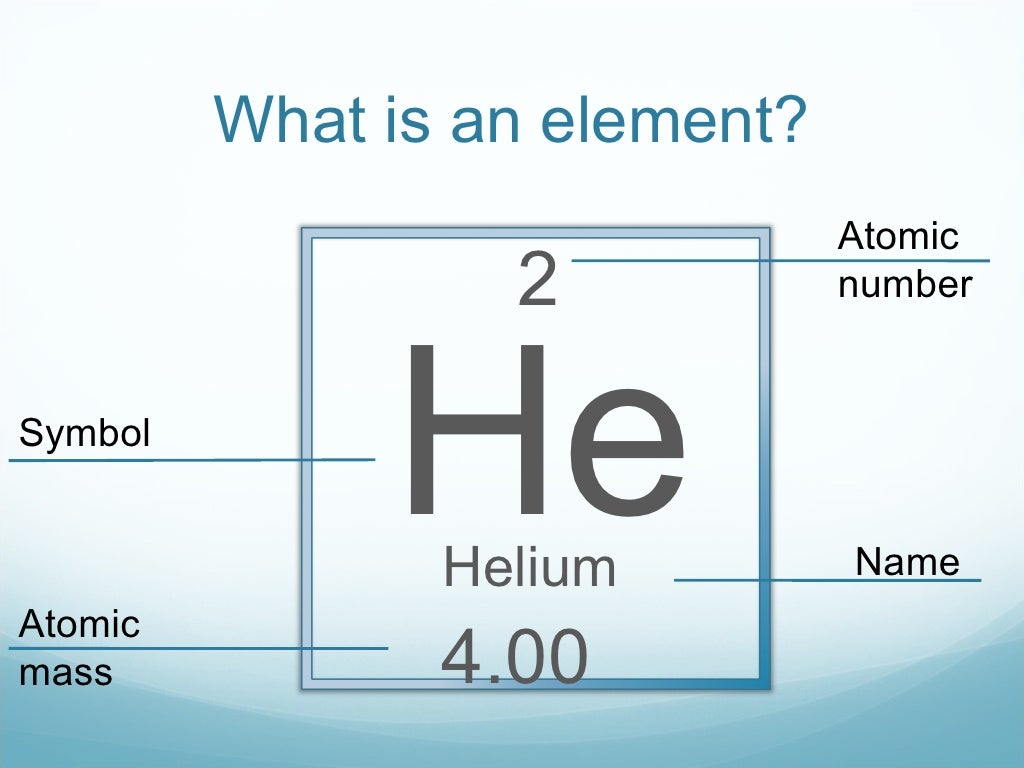
It is useful to know what the nuclear symbols are: Atomic Structure – Atomic and Mass Number Electrons have a negligible mass, so we don’t usually count it when working out the mass of an atom.
The mass numberis the total number of protons and neutron which are found in the nucleus. Each element has its own atomic number, characteristic of the element and helping you recognise it. The atomic number is the number of protons in the nucleus. This definition can be represented in an equation, as shown below. Atomic Structure - Atomic and Mass Number (A-Level Chemistry) Atomic and Mass Number Atomic and Mass Number Key Terms The mass number of an atom is equal to the total number of protons and neutrons contained in its nucleus.


 0 kommentar(er)
0 kommentar(er)
